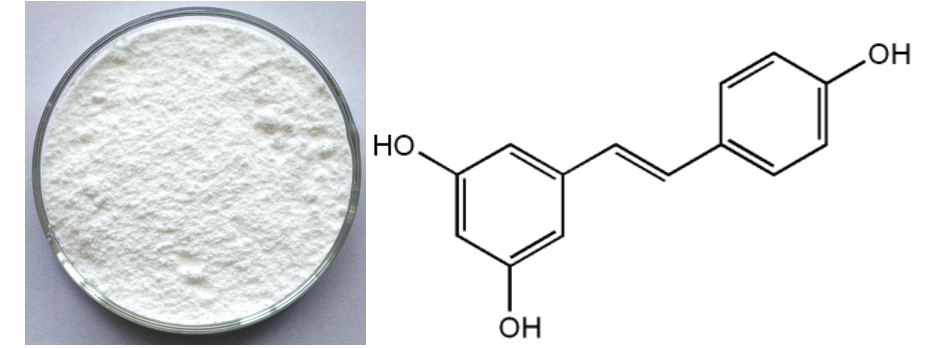
2017 wholesale price Resveratrol Factory from Melbourne
2017 wholesale price Resveratrol Factory from Melbourne Detail:
[Latin Name] Polygonum Cuspidatum Sieb. et Zucc
[Plant Source] China
[Specifications] Resveratrol 50%, 95%, 98% by HPLC
[Appearance]Brown or white fine powder
[Plant Part Used] Rhizome&Root
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Package] Packed in paper-drums and two plastic-bags inside.
[General feature]
1.100% natural source. Our resveratrol is 100% extracted from natural herb, very safe and more bioactive, which is rich with both CIS-resveratrol and trans-resveratrol.
2.Our resveratrol almost have no unpleasant taste compare to other resveratrols and it can be easier to take by oral.
3.We offer resveratrol at a very competitive price with superb quality.
4.We have a very large output and could manufacturer as customer particular requirement.
[Function]
Resveratrol is an active component extracted from Huzhang (Polygonum cuspidatum) in China.
It is an antioxidant phenol and a potent vasodilator that inhibits serum triglyceride synthesis, lipid peroxidation, and platelet aggregation.
It is extensively used for treatment of blood vessel disease such as atherosclerosis and hyperlipidemia. In addition, it has anti-virus and anti inflammatory activity, can treat acute microbial infections and viral hepatitis.
Product detail pictures:

Related Product Guide:
Adhering into the theory of "quality, services, efficiency and growth", now we have gained trusts and praises from domestic and international shopper for 2017 wholesale price Resveratrol Factory from Melbourne , The product will supply to all over the world, such as: Slovakia, India, Finland, All these products are manufactured in our factory located in China. So we can guarantee our quality seriously and availably. Within these four years we sell not only our products but also our service to clients throughout the world.
Created by Ryan Scott Patton.
Watch the next lesson: https://www.khanacademy.org/test-prep/mcat/chemical-processes/amino-acids-peptides-proteins-5d/v/central-dogma-of-molecular-biology-2?utm_source=YT&utm_medium=Desc&utm_campaign=mcat
Missed the previous lesson? https://www.khanacademy.org/test-prep/mcat/chemical-processes/nucleic-acids-lipids-and-carbohydrates/v/keto-enol-tautomerization?utm_source=YT&utm_medium=Desc&utm_campaign=mcat
MCAT on Khan Academy: Go ahead and practice some passage-based questions!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We’ve also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s MCAT channel: https://www.youtube.com/channel/UCDkK5wqSuwDlJ3_nl3rgdiQ?sub_confirmation=1
Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy
Di lang Breast Cancer ni Maritoni Fernandez, o Prostate Cancer ni April Boy Regino and pwedeng tulungan ng Pure Young Organic Barley Grass kungdi halos lahat ng karamdaman o simpleng prevention pang araw-araw, kung may tanong at order, tawag o text sa 09086449898 smart, 09058351552, tm, at 09225933577 sun.
The manufacturer gave us a big discount under the premise of ensuring the quality of products, thank you very much, we will select this company again.