High reputation for Ginseng extract in Venezuela
High reputation for Ginseng extract in Venezuela Detail:
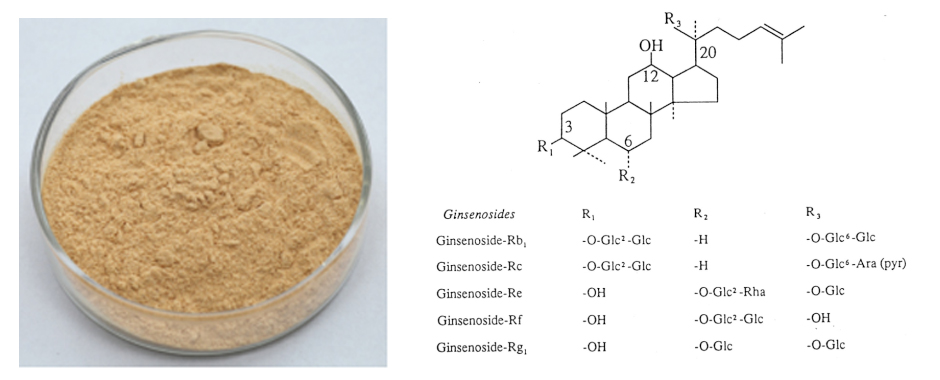
[Latin Name] Panax ginseng CA Mey.
[Plant Source] Dried Root
[Specifications] Ginsenosides 10%–80%(UV)
[Appearance] Fine Light Milk Yellow Powder
[Particle size] 80 Mesh
[Loss on drying] ≤ 5.0%
[Heavy Metal] ≤20PPM
[Extract solvents] Ethanol
[Microbe] Total Aerobic Plate Count: ≤1000CFU/G
Yeast & Mold: ≤100 CFU/G
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life]24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[What is Ginseng]
In terms of modern scientific research, ginseng is known to be an adaptogen. Adaptogens are substances that assist the body to restore itself to health and work without side effects even if the recommended dose is widely exceeded.
Ginseng due to its adaptogens effects is widely used to lower cholesterol, increase energy and endurance, reduce fatique and effects of stress and prevent infections.
Ginseng is one of the most effective antiaging supplements. It can alleviate some major effects of aging, such as degeneration of the blood system, and increase mental and physical capacity.
Other important benefits of ginseng is its support in cancer treatment and its effects on sports performance.
[Application]
1. Applied in food additives, it owns the effect of antifatigue, anti-aging and nourishing brain;
2. Applied in pharmaceutical field, it is used to treat coronary heart disease, angina cordis, bradycardia and high heart rate arrhythmia, etc.;
3. Applied in cosmetics field, it owns the effect of whitening, dispelling spot, anti-wrinkle, activating skin cells, making skin more tender and firm.
Product detail pictures:

Related Product Guide:
Good quality To start with,and Purchaser Supreme is our guideline to offer the top service to our customers.Presently, we've been seeking our best to be amongst the top exporters inside our industry to fulfill consumers extra need to have for High reputation for Ginseng extract in Venezuela , The product will supply to all over the world, such as: Uruguay, Nepal, Liverpool, Our company now has many department, and there have more than 20 employees in our company. We set up sales shop, show room, and product warehouse. In the meantime, we registered our own brand. We've got tightened inspection for quality of product.
Expand your vocabulary and learn how to say new words:
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia_Sinensis.html
Please leave a Like, a Comment, and Share.
Bookmark us and share:
https://www.dictionaryvoice.com
Twitter:
https://twitter.com/DictionaryVoice
Facebook:
https://www.facebook.com/pages/Dictionary-Voice/750369141710497
More Pronunciations:
1) How to Pronounce Camellia Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia_Sinensis.html
2) How to Pronounce Camellia
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia.html
3) How to Pronounce Centropus Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Centropus_Sinensis.html
4) How to Pronounce Alligator Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Alligator_Sinensis.html
5) How to Pronounce Camellia Two
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia_Two.html
6) How to Pronounce Primula Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Primula_Sinensis.html
7) How to Pronounce Clonorchis Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Clonorchis_Sinensis.html
8) How to Pronounce Camellia Japonica
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia_Japonica.html
9) How to Pronounce Lost Camellia
https://www.dictionaryvoice.com/How_To_Pronounce_Lost_Camellia.html
10) How to Pronounce Davidoff Camellia
https://www.dictionaryvoice.com/How_To_Pronounce_Davidoff_Camellia.html
11) How to Pronounce Citrus Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Citrus_Sinensis.html
12) How to Pronounce Sophora Sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Sophora_Sinensis.html
13) How to Pronounce Camellia State
https://www.dictionaryvoice.com/How_To_Pronounce_Camellia_State.html
14) How to Pronounce Lost-camellia
https://www.dictionaryvoice.com/How_To_Pronounce_Lost-camellia.html
15) How to Pronounce Hibiscus Rosa-sinensis
https://www.dictionaryvoice.com/How_To_Pronounce_Hibiscus_Rosa-sinensis.html
Descrizione
We are really happy to find such a manufacturer that ensuring product quality at the same time the price is very cheap.