2 Years\\\\\\\’ Warranty for Huperzine A Manufacturer in Angola
2 Years\\\\\\\’ Warranty for Huperzine A Manufacturer in Angola Detail:
[Latin Name]Huperzia serratum
[Source] Huperziceae whole herb from China
[Appearance]Brown to white
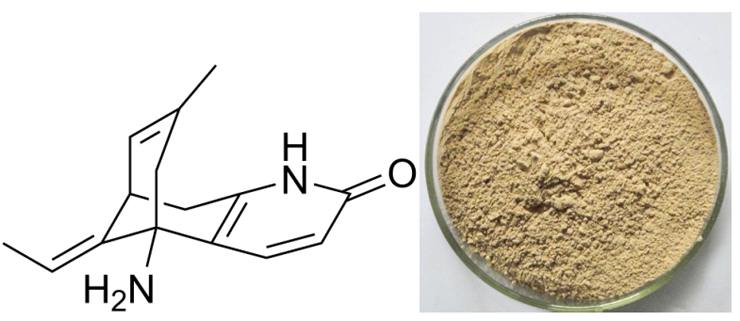
[Ingredient]Huperzine A
[Specification]Huperzine A 1% – 5%, HPLC
[Solubility] Soluble in chloroform, methanol, ethanol, slightly soluble in water
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Pesticide residue] EC396-2005, USP 34, EP 8.0, FDA
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[What is Huperzine A]
Huperzia is a type of moss that grows in China. It is related to club mosses (the Lycopodiaceae family) and is known to some botanists as Lycopodium serratum . The whole prepared moss was used traditionally. Modern herbal preparations use only the isolated alkaloid known as huperzine A. Huperzine A is an alkaloid found in huperzia that has been reported to prevent the breakdown of acetylcholine, an important substance needed by the nervous system to transmit information from cell to cell. Animal research has suggested that huperzine A’s ability to preserve acetylcholine may be greater than that of some prescription drugs. Loss of acetylcholine function is a primary feature of several disorders of brain function, including Alzheimer’s disease . Huperzine A may also have a protective effect on brain tissue, further increasing its theoretical potential for helping reduce symptoms of some brain disorders.
[Function] Used in alternative medicine, huperzine A has been found to act as a cholinesterase inhibitor, a type of medicine used to prevent the breakdown of acetylcholine (a chemical essential to learning and memory).
Not only used as a treatment for Alzheimer’s disease, huperzine A is also said to enhance learning and memory and to protect against age-related cognitive decline.
In addition, huperzine A is sometimes used to boost energy, increase alertness, and aid in the treatment of myasthenia gravis (an autoimmune disorder that affects the muscles).
Product detail pictures:

Related Product Guide:
We intention to see quality disfigurement within the creation and supply the ideal support to domestic and overseas buyers wholeheartedly for 2 Years\\\\\\\’ Warranty for Huperzine A Manufacturer in Angola , The product will supply to all over the world, such as: Nepal, Greece, Cyprus, Ought to any of these products be of curiosity to you, remember to allow us to know. We are going to be satisfied to give you a quotation on receipt of one's in depth specs. We've our private experienced R&D enginners to meet any of one's requriements, We appear forward to receiving your enquires soon'and hope to have the opportunity to work together with you in the future. Welcome to check out our company.
Noni fruit, used to make noni juice and powdered concentrates, can be a valuable dietary supplement known for its cleansing, pain relieving and antibacterial properties.
Extensively used by the Polynesian people for its many diverse health enhancing properties, the entire noni tree is considered an extremely valuable and versatile therapeutic plant species in which all parts of the tree, including the leaves, roots and stems, as well as the fruits, have been utilized for over 2,000 years among many cultures throughout the South Pacific.The fruits, containing a broad range of active compounds, are generally the most popular part consumed and used topically as a medicinal fruit and tonic beverage with multiple medical uses for treating a wide variety of conditions. Learn more about this superfruit variety and how to make your own fermented or fresh juice.
Mountain Rose Herbs, Organic Noni Powder – https://bit.ly/2ggS5uO
Raw Unpasteurized Fermented 100% Pure Noni Juice Hawaiian Blessings- 32oz – https://amzn.to/2ggVwBH
Certified Organic Wildcrafted Noni Powder , Raw Food World,16oz – https://amzn.to/1JrpNHC
Genesis Today Organic NONI 100 — 32 fl oz – https://amzn.to/1WY9U2u
Nutramedix Noni Extract (Morinda citrifolia) 1oz – https://amzn.to/2fSyzYA
Starwest Botanicals, Org Noni Powder, 4oz-1lb – https://bit.ly/2f7VVdC
Live Superfoods Noni Powder, Raw Organic, 16oz – https://bit.ly/2fSyRPa
Noni Fruit Page: https://bit.ly/1LEF8sf
Additional Sourced Info:
Evaluation of the ergogenic potential of noni juice: https://1.usa.gov/1LHMAmh
Immunostimulant activity of noni (Morinda citrifolia) on T and B lymphocytes: https://1.usa.gov/1NMSF0f
Effect of Noni (Morinda citrifolia Linn.) Fruit and Its Bioactive Principles Scopoletin and Rutin: https://1.usa.gov/1VjNvep
Analgesic and antiinflammatory activity of Morinda citrifolia L. (Noni) fruit: https://1.usa.gov/1F4Exs1
Anti-fungal activity of Morinda citrifolia (noni) extracts against Candida albicans: an in vitro study: https://1.usa.gov/1LHMDi1
Antitumor activity of fermented noni exudates and its fractions: https://1.usa.gov/1JogKVY
Anti-cancer Activity of Noni Fruit Juice Against Tumors in Mice: https://bit.ly/1UiViH2
An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumour activity: https://1.usa.gov/1Eo3nIv
The iridoid glycoside, asperulosidic acid in Morinda fruit extract was shown to increase blood flow in one 2014 study: https://1.usa.gov/1JogLsM
All information is for educational purposes only and is the personal view of the author; not intended as medical advice,
diagnosis or prescription. This information has not been evaluated by the FDA and is not intended to cure or prevent any disease.
Trà Xanh và Nấm Linh Chi là hai dược liệu quí trong tự nhiên giúp ngăn chặn tế bào ung thư, mà không làm tổn hại đến các tế bào lành.
Đây là một trong các video phóng sự được làm tại Đài Loan, ghi nhận các trường hợp thực tế đã điều trị thành công bệnh Ung thư bằng cách kết hợp các phương pháp hiện có với Cao Khô Linh Chi Đỏ Reishimax & Chiết Xuất Trà Xanh Tegreen’97. Reishimax và Tegreen97 là 2 sản phẩm đã được chứng minh hiệu quả tốt trên lâm sàng và đã được đưa vào danh mục thuốc điều trị tại liên bang Mỹ – The Federal Physical Desk Reference (PDR.net)
Bạn hãy chia sẻ cho thật nhiều người biết để giúp họ hoặc người thân có thêm phương pháp thiết thực thoát khỏi căn bệnh quái ác này nhé.
Để biết thêm chi tiết về cơ chế tác dụng xin vui lòng liên hệ qua email songtresongkhoe@gmail.com
More and more proofs of EGCG in green tea and Polysacchride in Lingzhi help killing maglinent cells but protect healthy cells.
This is one of video reports from Taiwan, about successful testimonials of different type cancer patients who applied a combine treatment of current oncology plus high concentrate red Ganoderma Lucidum mushroom (Reishimax) and green tea high concentrate (Tegreen’97). We can trust the safety and effectiveness of Reishimax & Tegreen97 as they are listed in The Federal Physical Desk Reference (PDR.net)
Please share to more friends and beloves to help them get rid of this devil disease
For more details on treatment and dosage please contact through email songtresongkhoe@gmail.com
Problems can be quickly and effectively resolved, it is worth to be trust and working together.