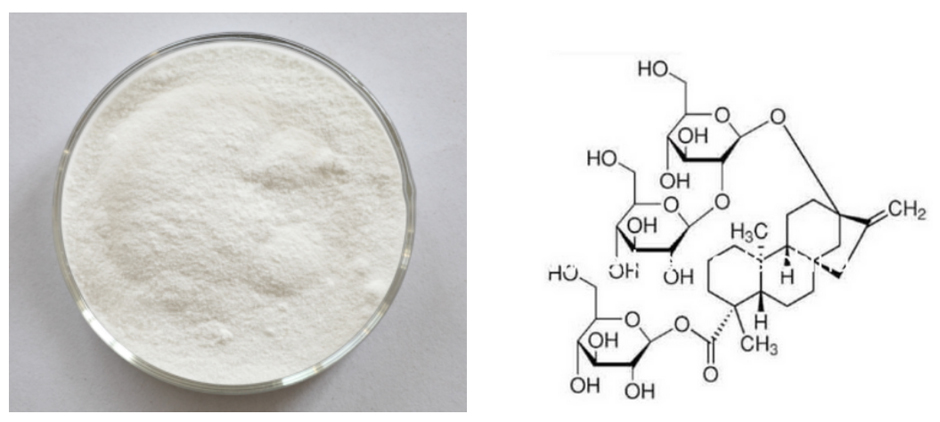
Hot-selling attractive price Stevia Extract Wholesale to Bahamas
Hot-selling attractive price Stevia Extract Wholesale to Bahamas Detail:
[Latin Name] Stevia rebaudiana
[Plant Source]from China
[Specifications] 1.Stevia Extract Powder (Steviosides)
Total Steviol Glycosides 80%, 90%, 95%
2. Rebaudioside-A
Rebaudioside-A 40%, 60%, 80%, 90%, 95%, 98%
3. Stevioside 90%
One monomer in Steviol Glycosides
[Appearance] Fine white powder
Plant Part Used:Leaf
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[Net weight] 25kgs/drum
Stevia Extract
[Characteristics]
Stevia sugar features high sweetness and low calorie and its sweetness is 200 350 times of that of cane sugar but its calorie is only 1/300 of that of cane sugar.
The component of stevia extract that gives it its sweetness is a mixture of various steviol glycosides. The components of sweetness in stevia leaves are stevioside, rebaudioside A, C, D, E and dulcoside A. Rebaudioside C, D, E and dulcoside A are small in quantity. The principal components are stevioside and rebaudioside A.
The quality of stevioside and rebaudiosideA is better than those of other components, which are commercially extracted and used in various applications.
The steviol glycosides present in stevia extract are referred to as “steviosides” or ¡°stevia extract¡±. Among these “steviosides”, the most common is Stevioside followed by RebaudiosideA. The Stevioside has a slight and pleasant herbal taste and the Rebaudioside-A has no herbal taste.
Although Rebaudioside C and dulcoside A are small in quantity in stevia extract, they are the major components giving bitter aftertaste.
[Function]
A large number of pharmaceutical tests have proved that stevia sugar has no side effects, carcinogens, and is safe for eating.
Compared with cane sugar, it can save 70% of the cost. With pure white color, pleasing taste and no peculiar smell, Stevia sugar is a new sugar source with broad perspective for development. Stevia rebaudianum sugar is the natural low hotsweet agent mostly similar to the flavor of cane sugar, approved to be used by State Ministry of Health and Ministry of Light Industry.
It is the third natural succedaneum of cane sugar and beet sugar with development and health care value, extracted from the leaves of the herbal vegetable of the composite family-stevia rebaudianum.
Product detail pictures:

Related Product Guide:
We rely upon strategic thinking, constant modernisation in all segments, technological advances and of course upon our employees that directly participate inside our success for Hot-selling attractive price Stevia Extract Wholesale to Bahamas , The product will supply to all over the world, such as: Lisbon, Milan, South Korea, we rely on own advantages to build a mutual-benefit commerce mechanism with our cooperative partners. As a result, we have gained a global sales network reaching the Middle East, Turkey, Malaysia and Vietnamese.
You can find more about herbal sexual enhancement pills for women at
https://www.naturalwomenhealth.com/natural-female-sexual-enhancement-pills.htm
Dear friend, in this video we are going to discuss about the herbal sexual enhancement pills for women. Kamni capsules are the best herbal sexual enhancement pills for women.
If you liked this video, then please subscribe to our YouTube Channel to get updates of other useful health video tutorials.
Herbal Sexual Enhancement Pills For Women
QUFU, China, Jan. 7, 2013 /PRNewswire/ — Sunwin Stevia International, Inc. “Sunwin Stevia” (SUWN) one of the top global providers of high quality stevia extracts including Rebaudioside A 98, announced today that its facilities are now capable of producing A3-99 stevia products. The addition of A3-99 to its commercial production capabilities is part of its previously announced stevioside expansion project.
A3-99 extracts are among the highest quality stevioside extracts produced in the world and are used in the pharmaceutical and food and beverage industries. Sunwin Stevia has completed testing for this production process making it one of a select few producers of A3-99 in China. Management expects to begin delivering shipments of the A3-99 to Zhejiang Huisong Pharmaceuticals, a pharmaceutical customer in China, in the fourth quarter of fiscal 2013 which begins in February 2013. Sunwin also intends to seek patents in China for its A3-99 production methodology.
“We are excited to add A3-99 to our commercial product offerings making Sunwin Stevia one of a select few suppliers capable of producing this extract on a large scale. Our recent facility upgrade has substantially increased our overall capabilities and we anticipate further additions to our production capabilities as we continue testing and trial production runs,” commented Dongdong Lin, CEO of Sunwin Stevia. “We believe our technology is state of the art and we intend to seek a patent for our methodologies. We look forward to actively marketing our A3-99 stevia in China and eventually in the international markets in the coming quarters.”
About Sunwin Stevia International, Inc.
Sunwin Stevia International, Inc. engages in the areas of zero calorie, all natural sweeteners (Sunwin Stevia™ Extracts). As an industry leader in agricultural processing, Sunwin has built an integrated global firm with the sourcing and production capabilities to meet the needs of consumers throughout the world. For more info about Sunwin, please visit https://www.sunwininternational.com
Safe Harbor Statement
Sunwin Stevia International, Inc. is hereby providing cautionary statements identifying important factors that could cause our actual results to differ materially from those projected in forward-looking statements (as defined in such act). Any statements that are not historical facts and that express, or involve discussions as to, expectations, beliefs, plans, objectives, assumptions or future events or performance (often, but not always, indicated through the use of words or phrases such as “will likely result,” “are expected to,” “will continue,” “is anticipated,” “estimated,” “intends,” “plans,” “believes” and “projects”) may be forward-looking and may involve estimates and uncertainties which could cause actual results to differ materially from those expressed in the forward-looking statements. These statements include, but are not limited to, our ability to obtain patents, future production capabilities, our technology and the market for our products, and other risk factors impacting our company, some of which may be beyond our control. We caution that the factors described herein could cause actual results to differ materially from those expressed in any forward-looking statements we make and that investors should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which such statement is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of anticipated or unanticipated events or circumstances. New factors emerge from time to time, and it is not possible for us to predict all of such factors. Further, we cannot assess the impact of each such factor on our results of operations or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. This press release is qualified in its entirety by the cautionary statements and risk factor disclosure contained in our Securities and Exchange Commission filings, including our Annual Report on Form 10-K for the fiscal year ended April 30, 2012.
Contact:
Dore Perler
U.S. Representative
954-232-5363
ir@sunwininternational.com
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.