Trending Products Dandelion root extract Wholesale to Japan
Trending Products Dandelion root extract Wholesale to Japan Detail:
[Latin Name] Taraxacum officinale
[Plant Source] from China
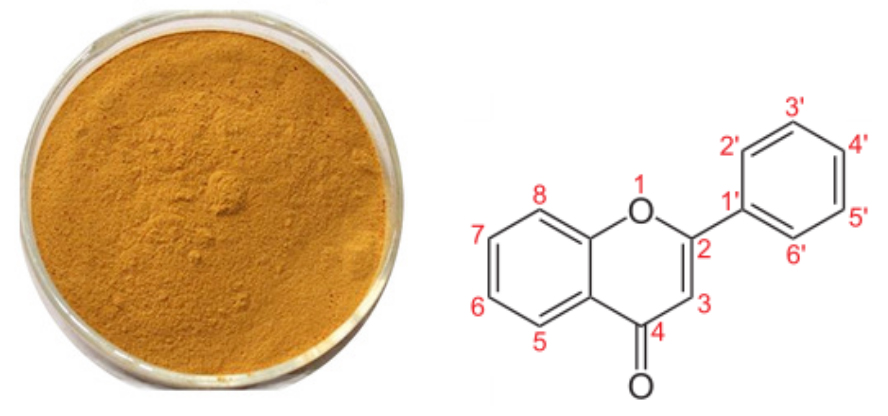
[Specifications] Flavones 3%-20%
[Appearance] Brown fine powder
Plant Part Used:Root
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[Net weight] 25kgs/drum
[Function]
(1) It is a general stimulant to the system, but especially to the urinary organs, and is chiefly used in kidney and liver disorders;
(2) Dandelion is also used as a remedy for hemorrhoids, gout, rheumatism, eczema, other skin conditions, and diabetes.
(3) Dandelion is used to treat chronic ulcers, stiff joints, and tuberculosis. It is also used to induce milk production in nursing mothers and to soothe inflamed breast tissue.
[Pharmacological effects]
(1) the antibacterial action: made of injection to extract the dandelion staphylococcus aureus and have strong hemolytic streptococcus pneumoniae, to kill, meningococci, diphtheria bacili, pseudomonas aeruginosa, proteus, dysenteric bacili, typhoid bacillus and card he also must kill staphylococcus, fungi, viruses, and some of the leptospira bacterium.
(2)other function. Advantageous bravery,diuresis and bitter soa, mild diarrhea inferior.
[Applications]
Dandelions extract injection, decoction, tablet, syrup, etc for a variety of infection are dampness.the curative effects, including the upper respiratory tract infection and chronic bronchitis, pneumonia, contagious hepatitis, urinary tract infection, surgical disorders, surgery, dermatology inflammation and sepsis inflammation, typhoid, biliary feeling, mumps, etc.
Product detail pictures:

Related Product Guide:
To create much more benefit for consumers is our company philosophy; customer growing is our working chase for Trending Products Dandelion root extract Wholesale to Japan , The product will supply to all over the world, such as: Indonesia, Jordan, Washington, We win many reliable customers by rich experience, advanced equipments, skilled teams, strict quality control and best service. We can guarantee all our products. Customers' benefit and satisfaction are always our biggest goal. Please contact us. Give us a chance, give you a surprise.
https://penis-enlargement-pills-and-device.com/VirilityEX
Virility Ex male enhancement pill for greater sexual performances. Virility EX has natural ingredients long used to address problems of enhancement………
Have Pineapple And Turmeric Beverage To Prevent Cancer, Beat Inflammation And Cold Too!
Lemon, ginger, turmeric and pineapple are the four most potent natural foods which fight inflammation and tumors. They are extremely healthy and provide numerous health benefits.
The following mixture is incredibly beneficial and it treats inflammation, common colds, and prevents cancer!
Here are some of the health benefits of its ingredients:
Lemon
The citric acid in lemons dissolves kidney stones. Researchers have found that these fruits contain 22 compounds which fight cancer and lower the cancer risk by 50%!
Lemons are also high in vitamin C, potassium, fibers, vitamin B6, and folate, and thus are helpful in the treatment of various health problems.
Ginger
Ginger contains gingerols, shogaols and paradols, which have potent anticancer properties.
Turmeric
Its active ingredient, curcumin, fights cancer, and it has been found to be even more effective than conventional cancer medicines.
Pineapple
Pineapples are rich in magnesium, manganese, bromelain, vitamin C, and B1, B5 and B6, and they help weight loss, detox the body, and treat a sore throat and a cough.
Black Pepper and Coconut
These two boost the absorption of nutrients.
This is how to prepare this remedy:
Ingredients:
1 lemon peeled
2 inches ginger
3-inch turmeric
2 cups pineapple
½ teaspoon pepper
1 teaspoon coconut oil
Instructions:
You should mix all ingredients in a blender, except the coconut oil. When you get a smooth mixture, add it and stir well.
Use:
Consume the remedy within 15 minutes after it is ready to enjoy all its benefits.
The supplier abide the theory of "quality the basic, trust the first and management the advanced" so that they can ensure a reliable product quality and stable customers.