Best quality and factory Citrus Aurantium Extract Factory for Namibia
Best quality and factory Citrus Aurantium Extract Factory for Namibia Detail:
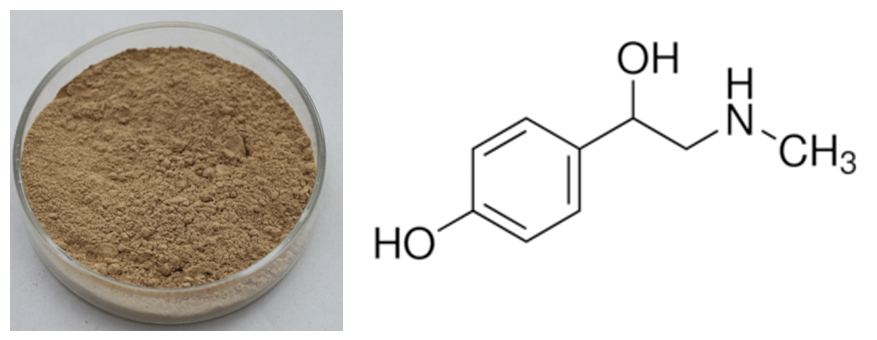
[Latin Name] Citrus aurantium L.
[Specification] Synephrine 4.0%–80%
[Appearance] Yellow brown powder
Plant Part Used: Fruit
[Particle size] 80Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[Net weight] 25kgs/drum
[What is Citrus Aurantium]
Citrus aurantium L, belonging to the family Rutaceae, is widely distributed in China. Zhishi, the Chinese traditional name for Citrus aurantium, has long been a folk medicine in traditional Chinese medicine (TCMto improve indigestion and help stimulate the Qi (energy force).
[Function]
1. Have the function of antioxidant, anti-inflammatory, hypolipidemic, vasoprotective and anticarcinogenic and cholesterol lowering actions.
2. Have the function of inhibiting following enzymes: Phospholipase A2, lipoxygenase, HMG-CoA reductase and cyclo-oxygenase.
3. Have the function of improving the health of capillaries by reducing the capillary permeability.
4. Have the function of reducing hay fever and other allergic conditions by inhibiting the release of histamine from mast cells. The possible activity of hesperidin could be explained by the inhibition of polyamine synthesis. (bitter orange extract)
Product detail pictures:

Related Product Guide:
Dedicated to strict high-quality management and considerate shopper company, our experienced team associates are normally available to discuss your requirements and ensure full shopper gratification for Best quality and factory Citrus Aurantium Extract Factory for Namibia , The product will supply to all over the world, such as: Italy, Sacramento, Luxembourg, All our staffs believe that: Quality builds today and service creates future. We know that good quality and the best service are the only way for us to achieve our customers and to achieve ourselves too. We welcome customers all over the word to contact us for future business relationships. Our products are the best. Once Selected, Perfect Forever!
Source:
https://www.spreaker.com/user/ms.bonita/rain-international-w-ms-b-seed-based-nut
https://www.myrainlife.com/ms.b
https://www.facebook.com/rainwithmsb/
Rain International w/ Ms.B – Seed Based Nutrition World Leader
Rain Soul : Post Convention Webinar SOUL
Dr Otto Heinrich Warburg (Germany)
Nobel Prize in Medicine 1931 : Biochemist
The body requires special fats that, among other important functions. make it possible for sufficient oxygen to reach the cells via the cellular membranes. Which are the key, These special fats are highly oxygen-absorbing. Called Essential Fatty Acids, or EFAs, these special fats must be supplied from outside the body every day.
Black raspberry seed :
Contain antioxidant-like phytonutrients including ellagitannins and anthocyanins. These may help fight viruses, inflammation, and a number of other serious health conditions. Antioxidants seek out and eliminate cell-damaging substances, called free radicals. Free radicals can occur naturally in the body or from exposure to environmental toxins. Although ellagitannins exist in most berries, raspberries contain the the most potent levels.
Black raspberry seed :
Contain antioxidant-like phytonutrients including ellagitannins and anthocyanins. These may help fight viruses, inflammation, and a number of other serious health conditions. Antioxidants seek out and eliminate cell-damaging substances, called free radicals. Free radicals can occur naturally in the body or from exposure to environmental toxins. Although ellagitannins exist in most berries, raspberries contain the the most potent levels.
Grape seed :
Grape seeds have an abundant source of flavonoids called proanthocyanidins. This is important for brain health due to their free radical-quenching antioxidant and collagen-protecting effects. Proanthocyanidins have also been shown to noticeably delay the onset of lipid peroxidation and to effectively chelate iron ions. Free iron ions are some of the most powerful promoters of lipid peroxide, hydrogen peroxide and hydroxyl radical production. This is a major contributor to the pathogenesis of Parkinsons disease, one of the most common neurological diseases in older individuals.
Ribose-D :
Ribose-D is a five-carbon sugar found in every cell in our bodies that combines with oxygen and ATP (adenosine triphoshate) to give energy to each cell. Ribose is also present in RNA (ribonucleic acid), which is one of the main information carriers of living organisms. Oxidative stress, as measured by free radical damage to cells, leads to systematic inflammation. Fortunately, our bodies handle this problem daily. However, if our bodies experience an abnormal increase in free radicals due to exercise, daily stress, excess smoking, excess saturated fat intake, depressed immune system, aging, etc., the body becomes fatigued and less efficient in producing Ribose-D to replenish cellular energy. Because of this, we need to supplement with Ribose-D in order to regenerate the cells energy system.
Grape Extracts Resveratrol :
Grape skin extract contains trans-resveratrol which is considered a natural antibiotic that can fight bacterial diseases. Resveratrol may help protect the body from various diseases and slowing down the aging process.
http;//ksmdherbs.com
This powerful formula works wonders and provides the confidence you need to support enhanced performance and stamina. There are many male sexual enhancement products available in the market and many of them claim that they are the best, the most effective and the safest
subscribe to our channel
Customer service staff and sales man are very patience and they all good at English, product's arrival is also very timely, a good supplier.