Good Quality for Lyophilized royal jelly powder Supply to Algeria
Good Quality for Lyophilized royal jelly powder Supply to Algeria Detail:
[Products Name] Royal jelly powder,Lyophilized royal jelly powder
[Specification] 10-HDA 4.0%, 5.0%, 6.0%, HPLC
[Gerneral feature]
1. Low antibiotics, Chloramphenicol< 0.1ppb
2.Organic certified by ECOCERT, according to EOS & NOP organic standard;
3.100% pure with no additives;
4. More easily absorbed into the body than fresh royal jelly
5. Can be easily produced into tablets.
[Our advantages]
- 600 bee farmers, 150 units of bee-feeding groups located in natural mountains;
- Organic certificated by ECOCERT;
- NON-antibiotics, widely exported to Europe;
- Health Certificate, Sanitary Certificate and Quality Certificate are available.
[Lyophilized technology]
Lyophilized technology, also known as Freeze-drying, it is a dehydration process typically used to maintain activity of all nutrition ingredients in royal jelly, also to make the royal jelly convenient for transport. Freeze-drying works by freezing the material and then reducing the surrounding pressure to allow the frozen water in the material to sublimate directly from the solid phase to the gas phase. This technology can maintain all activity of nutrition ingredient.
Lyophilized royal jelly powder is processed directly from fresh royal jelly.
3kgs fresh royal jelly is used to make 1kg lyophilized royal jelly powder.
During all the production process, there is no additives.
[Packing]
5kg/bag, 25kgs/drum
1kg/bag, 20kgs/carton
Main indices of physical and chemic in Lyophilized royal jelly
| Ingredients Indices | Lyophilized royal jelly | Standards | Results |
| Ash | 3.2 | <5 | Complies |
| Water | 4.1% | <7% | Complies |
| Glucose | 43.9% | <50% | Complies |
| Protein | 38.29% | >33% | Complies |
| 10-HDA | 6.19% | >4.2% | Complies |
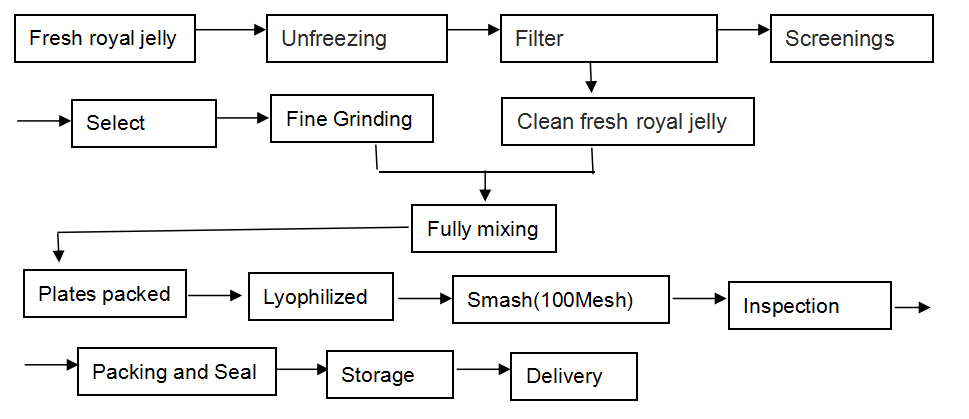
[Our work flow]
Our Lyophilized Royal Jelly Powder is produced in this way: we lyophilize the fresh royal jelly by advanced freeze-drying facilities without losing any nutritional ingredients, reserving the natural ingredients in utmost, and then make them into the form of powder, for any food additives are not needed to add.
The raw material we use is the natural fresh royal jelly which is up to the export standard . We process our products strictly according to export standard. Our workshop is up to the requirements of GMP.
Royal Jelly powder has been selected as drug excipients by many European and American pharmaceutical producing enterprises.Meanwhile it is applies to health food and cosmetics industries.
[Quality control]
Traceability record
GMP standard production
Advanced inspection equipment
[Function]
1.Enhances the immune system
2.Promotes wound healing
3.Has antitumor/anticancer properties
4.Lowers cholesterol levels
5.Increases fat metabolism
6.Is a powerful antioxidant
7.Regulates blood sugar levels
[Applications]
It’s widely used in health tonic, health pharmacy, hairdressing and cosmetic area, and mainly was applied in capsules, troche and oral liquids etc.
Product detail pictures:

Related Product Guide:
Our company insists all along the quality policy of "product good quality is base of enterprise survival; buyer fulfillment will be the staring point and ending of an company; persistent improvement is eternal pursuit of staff" and also the consistent purpose of "reputation very first, shopper first" for Good Quality for Lyophilized royal jelly powder Supply to Algeria , The product will supply to all over the world, such as: Greek, Panama, United Arab Emirates, With a wide range, good quality, reasonable prices and stylish designs, our items are extensively used in this field and other industries. We welcome new and old customers from all walks of life to contact us for future business relationships and achieving mutual success! We welcome customers, business associations and friends from all parts of the world to contact us and seek cooperation for mutual benefits.
Trà Xanh và Nấm Linh Chi là hai dược liệu quí trong tự nhiên giúp ngăn chặn tế bào ung thư, mà không làm tổn hại đến các tế bào lành.
Đây là một trong các video phóng sự được làm tại Đài Loan, ghi nhận các trường hợp thực tế đã điều trị thành công bệnh Ung thư bằng cách kết hợp các phương pháp hiện có với Cao Khô Linh Chi Đỏ Reishimax & Chiết Xuất Trà Xanh Tegreen’97. Reishimax và Tegreen97 là 2 sản phẩm đã được chứng minh hiệu quả tốt trên lâm sàng và đã được đưa vào danh mục thuốc điều trị tại liên bang Mỹ – The Federal Physical Desk Reference (PDR.net)
Bạn hãy chia sẻ cho thật nhiều người biết để giúp họ hoặc người thân có thêm phương pháp thiết thực thoát khỏi căn bệnh quái ác này nhé.
Để biết thêm chi tiết về cơ chế tác dụng xin vui lòng liên hệ qua email songtresongkhoe@gmail.com
More and more proofs of EGCG in green tea and Polysacchride in Lingzhi help killing maglinent cells but protect healthy cells.
This is one of video reports from Taiwan, about successful testimonials of different type cancer patients who applied a combine treatment of current oncology plus high concentrate red Ganoderma Lucidum mushroom (Reishimax) and green tea high concentrate (Tegreen’97). We can trust the safety and effectiveness of Reishimax & Tegreen97 as they are listed in The Federal Physical Desk Reference (PDR.net)
Please share to more friends and beloves to help them get rid of this devil disease
For more details on treatment and dosage please contact through email songtresongkhoe@gmail.com
Now that frosts are coming its time to harvest the stevia. I try to let mine stay out as long as possible since I have read that the sweetness intensifies with cooler temperatures.This is one of the easiest herbs to grow and dry.
We are long-term partners, there is no disappointment every time, we hope to maintain this friendship later!