Hot New Products Astaxanthin Factory in Surabaya
Hot New Products Astaxanthin Factory in Surabaya Detail:
[Latin Name] Haematococcus Pluvialis
[Plant Source] from China
[Specifications]1% 2% 3% 5%
[Appearance] Dark red Powder
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[Net weight] 25kgs/drum
Brief Introduction
Astaxanthin is a natural nutritional component, it can be found as a food supplement. The supplement is intended for human, animal, and aquaculture consumption.
Astaxanthin is a carotenoid. It belongs to a larger class of phytochemicals known as terpenes, which are built from five carbon precursors; isopentenyl diphosphate and dimethylallyl diphosphate . Astaxanthin is classified as a xanthophyll (originally derived from a word meaning “yellow leaves” since yellow plant leaf pigments were the first recognized of the xanthophyll family of carotenoids), but currently employed to describe carotenoid compounds that have oxygen-containing moities, hydroxyl or ketone , such as zeaxanthin and canthaxanthin. Indeed, astaxanthin is a metabolite of zeaxanthin and/or canthaxanthin, containing both hydroxyl and ketone functional groups. Like many carotenoids, astaxanthin is a colorful, lipid-soluble pigment. This colour is due to the extended chain of conjugated (alternating double and single) double bonds at the centre of the compound. This chain of conjugated double bonds is also responsible for the antioxidant function of astaxanthin (as well as other carotenoids) as it results in a region of decentralized electrons that can be donated to reduce a reactive oxidizing molecule.
Function:
1.Astaxanthin is a powerful antioxidant and may protect against oxidative damage to body tissues.
2.Astaxanthin can improve the immune response by increasing the number of antibody producing cells.
3.Astaxanthin is a potential candidate to treat neurodegenerative disease such as Alzhimer and Parkinson diease.
4.Astaxanthin dan reduce UVA-light damage to skin such as sunburn, inflammation, ageing and skin cancer.
Application
1.When applied in pharmaceutical field, astaxanthin powder has the good function of antineoplastic;
2.When applied in health food field, astaxanthin powder is used as food additives for pigment and health care;
3.When applied in cosmetic field, astaxanthin powder has the good function of antioxidant and anti-aging;
4.When applied in animal feeds field, astaxanthin powder is used as animal feed additive to impart coloration, including farm-raised salmon and egg yolks.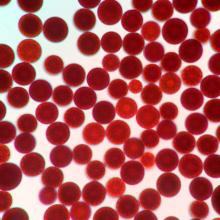
Product detail pictures:

Related Product Guide:
Our personnel are always inside the spirit of "continuous improvement and excellence", and together with the outstanding excellent goods, favorable price and good after-sales services, we try to gain every customer's trust for Hot New Products Astaxanthin Factory in Surabaya , The product will supply to all over the world, such as: Melbourne, Iceland, Paraguay, Welcome to visit our company, factory and our showroom where displays various hair products that will meet your expectation. Meanwhile, it is convenient to visit our website, and our sales staff will try their best to provide you the best service. Please contact us if you need more information. Our aim is to help customers realize their goals. We are making great efforts to achieve this win-win situation.
Benefits of Water: These are *All the Real Proven Benefits of Drinking Water You Actually Need to Know Right Now Today…
1. 0:10 Is Drinking Water Good for You: Food is the fuel our bodies use for everything that they do. Food gives us the building blocks our bodies need to move, build new muscles, and keep our brain going. That’s why nutrition is the most important thing for keeping your body healthy. Read on for some tips on nutrition that will make you feel better – benefits of drinking water in the morning.
2. 0:40 Alkaline Water Benefits: Eat more soy containing foods for healthy bones. Many of the soy foods which contain soy, contain a lot of calcium, or they are fortified with calcium. Magnesium and borron, which work with calcium for optimum bone health, are also found in soy foods. Soy foods are great for strong, healthy bones – water nutrition.
3. 1:10 Drinking Water Benefits: For better health, bring your own lunch to school or work. Packing your own lunch puts you in control of exactly what you are eating. It only takes 10 minutes or so to get a few meals prepared – water for health.
4. 1:40 Water Benefits: When possible, include more foods in your diet that are high in omega 3. Omega 3 is one of the good fats that has many benefits. It can help reduce pain and swelling in an injury. It is a great source of protection against cardiovascular disease and strokes. It also has some benefits in treating attention deficit disorders – benefit of water.
5. 2:10 Health Benefits of Water: Instead of reaching for coffee or an energy drink the moment that you wake up, turn to a grapefruit, apple or orange instead. Natural fruits are fantastic for your body because they come with a multitude of vitamins that are essential for your health and nutrition. Adding these to your routine, can also improve your energy level during the day – benefits of drinking lots of water.
6. 2:40 Benefits of Drinking Warm Water: Try to stay on course with meal times. If you get your child into a routine it will be much easier to get them to eat. The child will learn when he will eat next so he will feel more hungry at that time. Always offer fruits or vegetables with every meal – water health.
7. 3:10 Water Nutrition Facts: A great nutritional tip is to make sure you eat before and after your workouts. It’s important to eat before you work out because your body will need plenty of fuel. It’s also very important to eat within a half hour of lifting weights because it will help your muscles recover – water health benefits.
8. 3:40 The Benefits of Drinking Water: Liven up your sandwiches with raw vegetables and fruit. Raw vegetables and fruit add crunch to a sandwich. They also add taste and vitamins and minerals. To make sandwich preparation a snap always be sure to keep some veggies pre-sliced and ready to go in baggies in your fridge – benefits of drinking alkaline water.
9. 4:10 Benefits of Drinking a Gallon of Water a Day: Keep your refrigerator and freezer stocked with quick-to-prepare healthy convenience meals. If you know you have some healthy TV dinners at home, you are much less likely to run by the fast food place after a harried day at work. Convenience meals shouldn’t be a nutritional staple but they sure are nice to have occasionally – benefits of drinking a lot of water.
10. 4:40 Benefit of Drinking Water: Soybeans are practically miraculous in their nutritional value. They contain a moderate amount of carbohydrates, lots of good protein, and just enough fat to help you absorb the good phytochemicals like isoflavones and the omega-3 fatty acids. Eating more protein in the form of soy products will also help you avoid saturated fats and cholesterol – benefits of drinking more water.
11. 5:10 Health Benefits of Drinking Water: Take a whiff of the aroma from bananas, peppermint and apples. Some foods like those can help reduce your appetite. There are people who believe that smelling these can trick the body into believing that the person has eaten. Suppressing your appetite is one way to lose weight – water and health.
12. 5:40 Advantages of Drinking Water: When it comes to nutrition, you want to make sure you are constantly keeping an eye on the latest information available to you. With science always learning new things about what is healthy and unhealthy for you, you want to try your best to always, be informed. You never know, something that you think could be helping you today could actually end up harming you in the future, so try your best to stay informed.
13. 7:00 What Are the Benefits of Drinking Water: Proper nutrition can be easy if you know how! If you feel weak or depressed, make sure to give some of the things in this article a try! The food you eat can have a big impact on how you feel. Keep these things in mind when you go to the grocery store – benefits of drinking water for skin.
Replay: https://www.youtube.com/watch?v=kJc0ztt-GD8
Resource: https://en.wikipedia.org/wiki/Drinking_water
The company can keep up with the changes in this industry market, product updates fast and the price is cheap, this is our second cooperation, it's good.







