Short Lead Time for Curcuma Longa Extract Wholesale to Norway
Short Lead Time for Curcuma Longa Extract Wholesale to Norway Detail:
[Latin Name] Curcuma longa L.
[Plant Source] Root From India
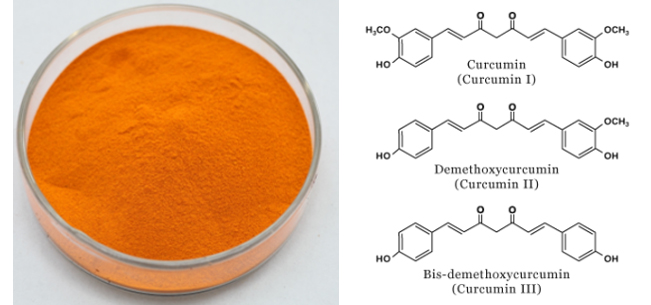
[Specification] Curcuminoids 95% HPLC
[Appearance] Yellow powder
Plant Part Used: Root
[Particle size]80Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Shelf life] 24 Months
[Package] Packed in paper-drums and two plastic-bags inside.
[Net weight] 25kgs/drum
[What is Curcuma Longa?]
Turmeric is an herbaceous plant known scientifically as Curcuma longa. It belongs to the Zingiberaceae family, which includes ginger. Tumeric has rhizomes rather than true roots, which are the primary source of commercial value for this plant. Tumeric originates from southwest India, where it has been a stable of Siddha medicine for thousands of years. It is also a common spice in Indian cuisine and is often used as flavoring for Asian mustards.
Product detail pictures:

Related Product Guide:
We have been committed to offering easy,time-saving and money-saving one-stop purchasing service of consumer for Short Lead Time for Curcuma Longa Extract Wholesale to Norway , The product will supply to all over the world, such as: Slovak Republic, Turin, Uganda, Our mission is to deliver consistently superior value to our customers and their clients. This commitment permeates everything we do, driving us to continuously develop and improve our products and the processes to fulfill your needs.
In 2009 the US FDA approved two versions of a new sweetener developed for Coca-Cola and Pepsi, Truvia and PureVia, both of which use rebiana—an extract from the South American plant Stevia. Truvia has just hit the market in Great Britain supported by gigantic public relations celebrations that would do an aspiring politician proud. Stevia, in its natural state, is the best no-calorie health-supporting natural sweetener on the planet. Here is what you should know:
1. Truvia is NOT Stevia.
2. I advise that you avoid it.
Like every plant which has long been used as a food source, Stevia is a complex of synergistic substances and compounds including numerous steviosides, rebaudiosides, and glycosides. It is this synergistic power that creates its unique benefits including its anti-oxidant properties. When the FDA declared that these two manufactured zero-calorie sweeteners were “generally recognized as safe” (GRAS) it referred only to a couple of the active ingredients taken from Stevia that had been used in manufacturing including rebaudioside A—a component which imparts to the natural Stevia plant some of its sweet taste.
What takes place when you extract only one or two of a plant’s parts and throw away the rest? Does the product you make out of this affect your body the same way as the natural plant? Virtually never. In The Toxicology of Rebaudioside A: A Review, researchers at UCLA report that a living organism metabolizes stevioside compounds and rebaudioside A at different rates. This makes it impossible to assess the potential risks to the body.
Click Here to view the Toxicology of Rebaudioside from the University of California
The company behind the development of both Truvia and PureVia is Cargill. Cargill is one of the most notorious corporate polluters in America (which Conde Nast Portfolio listed as one of the “Toxic Ten.”) Coke and Cargill have conducted their own ‘research’ into the safety of Truvia on which the FDA gave them the go ahead to sell the sweetener. However, no genuinely independent studies have been done to affirm the safety of the product.
And as even the Truvia website itself states: “While rebiana is natural and comes from a plant, it is not certified or grown organically at this time. That could happen in the future, depending on consumer demand.” How absurd. How can you claim to have invented an “all-natural” zero-cal sweetener that is not only not organically grown but which no genuinely independent studies have shown to be safe to use over time?
QUFU, China, Jan. 7, 2013 /PRNewswire/ — Sunwin Stevia International, Inc. “Sunwin Stevia” (SUWN) one of the top global providers of high quality stevia extracts including Rebaudioside A 98, announced today that its facilities are now capable of producing A3-99 stevia products. The addition of A3-99 to its commercial production capabilities is part of its previously announced stevioside expansion project.
A3-99 extracts are among the highest quality stevioside extracts produced in the world and are used in the pharmaceutical and food and beverage industries. Sunwin Stevia has completed testing for this production process making it one of a select few producers of A3-99 in China. Management expects to begin delivering shipments of the A3-99 to Zhejiang Huisong Pharmaceuticals, a pharmaceutical customer in China, in the fourth quarter of fiscal 2013 which begins in February 2013. Sunwin also intends to seek patents in China for its A3-99 production methodology.
“We are excited to add A3-99 to our commercial product offerings making Sunwin Stevia one of a select few suppliers capable of producing this extract on a large scale. Our recent facility upgrade has substantially increased our overall capabilities and we anticipate further additions to our production capabilities as we continue testing and trial production runs,” commented Dongdong Lin, CEO of Sunwin Stevia. “We believe our technology is state of the art and we intend to seek a patent for our methodologies. We look forward to actively marketing our A3-99 stevia in China and eventually in the international markets in the coming quarters.”
About Sunwin Stevia International, Inc.
Sunwin Stevia International, Inc. engages in the areas of zero calorie, all natural sweeteners (Sunwin Stevia™ Extracts). As an industry leader in agricultural processing, Sunwin has built an integrated global firm with the sourcing and production capabilities to meet the needs of consumers throughout the world. For more info about Sunwin, please visit https://www.sunwininternational.com
Safe Harbor Statement
Sunwin Stevia International, Inc. is hereby providing cautionary statements identifying important factors that could cause our actual results to differ materially from those projected in forward-looking statements (as defined in such act). Any statements that are not historical facts and that express, or involve discussions as to, expectations, beliefs, plans, objectives, assumptions or future events or performance (often, but not always, indicated through the use of words or phrases such as “will likely result,” “are expected to,” “will continue,” “is anticipated,” “estimated,” “intends,” “plans,” “believes” and “projects”) may be forward-looking and may involve estimates and uncertainties which could cause actual results to differ materially from those expressed in the forward-looking statements. These statements include, but are not limited to, our ability to obtain patents, future production capabilities, our technology and the market for our products, and other risk factors impacting our company, some of which may be beyond our control. We caution that the factors described herein could cause actual results to differ materially from those expressed in any forward-looking statements we make and that investors should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which such statement is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of anticipated or unanticipated events or circumstances. New factors emerge from time to time, and it is not possible for us to predict all of such factors. Further, we cannot assess the impact of each such factor on our results of operations or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. This press release is qualified in its entirety by the cautionary statements and risk factor disclosure contained in our Securities and Exchange Commission filings, including our Annual Report on Form 10-K for the fiscal year ended April 30, 2012.
Contact:
Dore Perler
U.S. Representative
954-232-5363
ir@sunwininternational.com
The quality of the products is very good, especially in the details, can be seen that the company work actively to satisfy customer's interest, a nice supplier.