OEM/ODM Supplier for Lyophilized royal jelly powder Factory from Spain
OEM/ODM Supplier for Lyophilized royal jelly powder Factory from Spain Detail:
[Products Name] Royal jelly powder,Lyophilized royal jelly powder
[Specification] 10-HDA 4.0%, 5.0%, 6.0%, HPLC
[Gerneral feature]
1. Low antibiotics, Chloramphenicol< 0.1ppb
2.Organic certified by ECOCERT, according to EOS & NOP organic standard;
3.100% pure with no additives;
4. More easily absorbed into the body than fresh royal jelly
5. Can be easily produced into tablets.
[Our advantages]
- 600 bee farmers, 150 units of bee-feeding groups located in natural mountains;
- Organic certificated by ECOCERT;
- NON-antibiotics, widely exported to Europe;
- Health Certificate, Sanitary Certificate and Quality Certificate are available.
[Lyophilized technology]
Lyophilized technology, also known as Freeze-drying, it is a dehydration process typically used to maintain activity of all nutrition ingredients in royal jelly, also to make the royal jelly convenient for transport. Freeze-drying works by freezing the material and then reducing the surrounding pressure to allow the frozen water in the material to sublimate directly from the solid phase to the gas phase. This technology can maintain all activity of nutrition ingredient.
Lyophilized royal jelly powder is processed directly from fresh royal jelly.
3kgs fresh royal jelly is used to make 1kg lyophilized royal jelly powder.
During all the production process, there is no additives.
[Packing]
5kg/bag, 25kgs/drum
1kg/bag, 20kgs/carton
Main indices of physical and chemic in Lyophilized royal jelly
| Ingredients Indices | Lyophilized royal jelly | Standards | Results |
| Ash | 3.2 | <5 | Complies |
| Water | 4.1% | <7% | Complies |
| Glucose | 43.9% | <50% | Complies |
| Protein | 38.29% | >33% | Complies |
| 10-HDA | 6.19% | >4.2% | Complies |
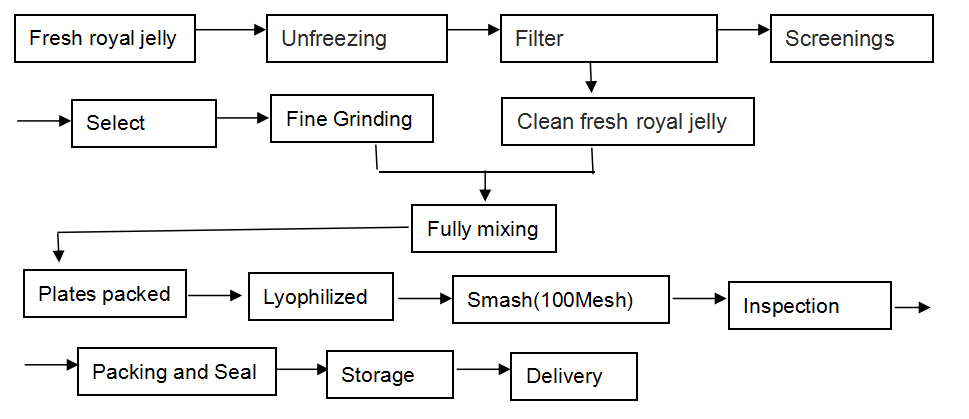
[Our work flow]
Our Lyophilized Royal Jelly Powder is produced in this way: we lyophilize the fresh royal jelly by advanced freeze-drying facilities without losing any nutritional ingredients, reserving the natural ingredients in utmost, and then make them into the form of powder, for any food additives are not needed to add.
The raw material we use is the natural fresh royal jelly which is up to the export standard . We process our products strictly according to export standard. Our workshop is up to the requirements of GMP.
Royal Jelly powder has been selected as drug excipients by many European and American pharmaceutical producing enterprises.Meanwhile it is applies to health food and cosmetics industries.
[Quality control]
Traceability record
GMP standard production
Advanced inspection equipment
[Function]
1.Enhances the immune system
2.Promotes wound healing
3.Has antitumor/anticancer properties
4.Lowers cholesterol levels
5.Increases fat metabolism
6.Is a powerful antioxidant
7.Regulates blood sugar levels
[Applications]
It’s widely used in health tonic, health pharmacy, hairdressing and cosmetic area, and mainly was applied in capsules, troche and oral liquids etc.
Product detail pictures:

Related Product Guide:
OEM/ODM Supplier for Lyophilized royal jelly powder Factory from Spain , The product will supply to all over the world, such as: , , ,
Title: Pneumococcal infections and vaccination.
4th International Conference on Vaccines & Vaccination
September 24-26, 2014 Valencia, Spain
OMICS International: https://omicsonline.org
Vaccine Conferences: https://conferenceseries.com/immunolog…
Global Medical Conferences: https://conferenceseries.com
Global Pharmaceutical Conferences: https://pharmaceuticalconferences.com
Global Cancer Conferences: https://cancersummit.org
Global Diabetes Conferences: https://diabetesexpo.com
Global Dental Conferences: https://dentalcongress.com
Global Nursing Conferences: https://nursingconference.com
Abstract
Pneumococci are major contributors to morbidity and mortality worldwide. They are the major cause of milder respiratory tract infections such as otitis and sinusitis, but also to more severe invasive infections such as community-acquired pneumonia with associated septicemia and meningitis. Even though being a devastating pathogen, pneumococci are also common colonizers of the upper respiratory tract of healthy children where from they may spread to cause disease. Risk groups for acquiring an invasive pneumococcal infection (IPD) include preschool children and the elderly, as well as immunocompromised individuals, splenectomised, and patients with a previous influenza virus infection. Several bacterial virulence factors have been described for pneumococci of which a major one is the capsular polysaccharide. Depending on differences in these capsular structures so far more than 90 different serotypes have been distinguished. Recently so called conjugated vaccines (PCV) have been introduced into the child hood vaccination program in many countries. These vaccines are based on a limited amount (7, 10 or 13) of the capsular serotypes. In Sweden PCVs were introduced in the whole of Sweden year 2009. Vaccine introduction has led to a reduction in the incidence of IPD in vaccinated children but also to serotype replacementand an increase of non-vaccine type disease.
Biography
Birgitta Henriques Normark is professor and head physician in Clinical microbiology at Karolinska Institute and Karolinska University hospital, as well as head physician at the Public Health Agency of Sweden. She is also vice dean for recruitment at Karolinska Institutet and a board member of the Swedish Research Council, Medicine and Health.Her research focuses on epidemiology, antibiotic resistance and host-microbe interactions in bacterial infections with special focus on pneumococcal epidemicity, molecular epidemiology, and mechanisms of invasive disease, innate immunity and host-microbe interactions determining disease outcome. The research has been published in 136 publications of which 107 are original peer reviewed articles.
1.1 cup Boiled Pumpkin
mesh well.
2.3tsp ghee
Cashews Almonds Raisins
fry for golden brown color
medium flame.
3.cook for 10 to 15mins
add 1/2cup sugar powder
1tsp corn flour
mix it well.
4.cook till halwa is
consistancy is reached.
low flame.
5.add cardamom powder
remove from the heat and
serve hot
6 ready
 By from -
By from -
 By from -
By from -